Overview
Streamlined development, evaluation and clinical translation of radiopharmaceuticals requires a partner that has extensive scientific, regulatory and operational expertise. Working with a CRO that provides a complete solution ensures data is delivered in a timely manner to make a go/no-go decision on your imaging or
therapeutic candidates.
Services Include:
- Single-site, first-in-human through multi-site, late phase clinical study support
- Investigational New Drug (IND)/Clinical Trial Application (CTA) submission support with clinical protocol development
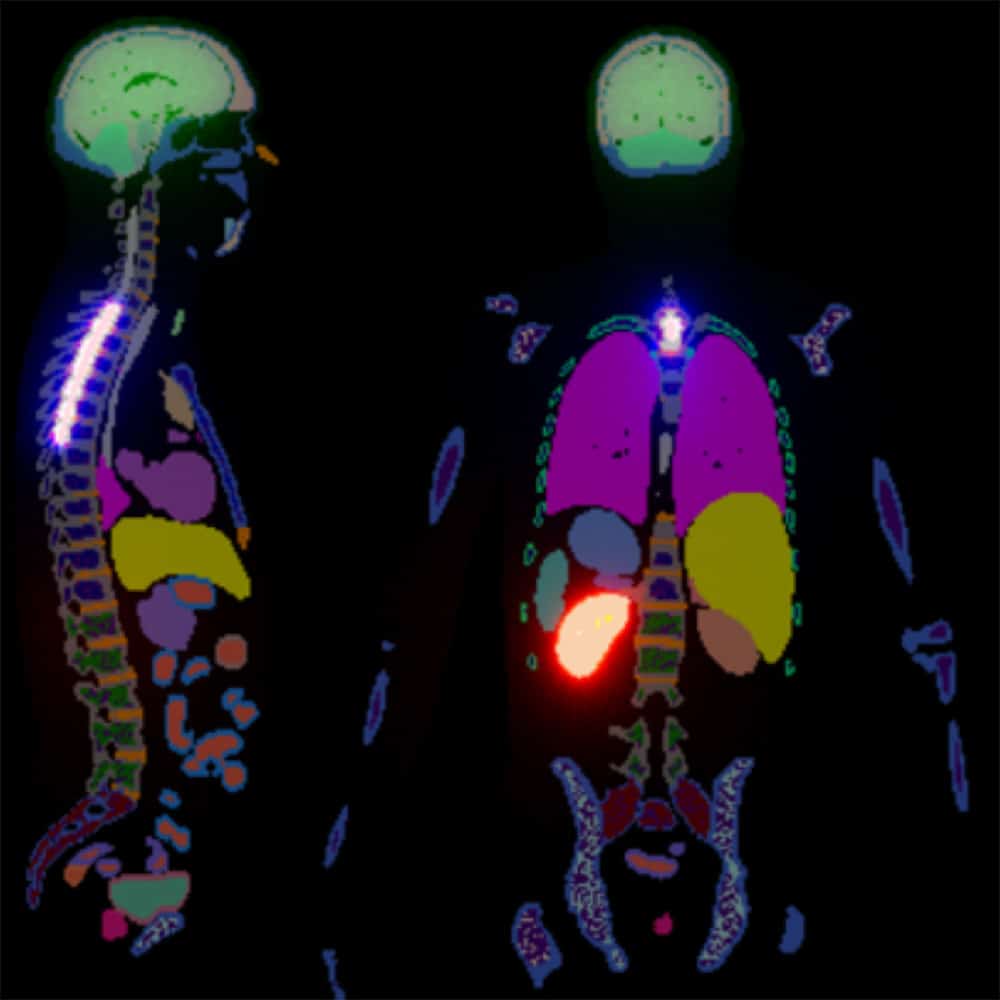
- Preclinical and clinical dosimetry and advanced quantitative image analysis
- Radiochemistry development – conjugation, radiolabeling, stability and immunoreactivity
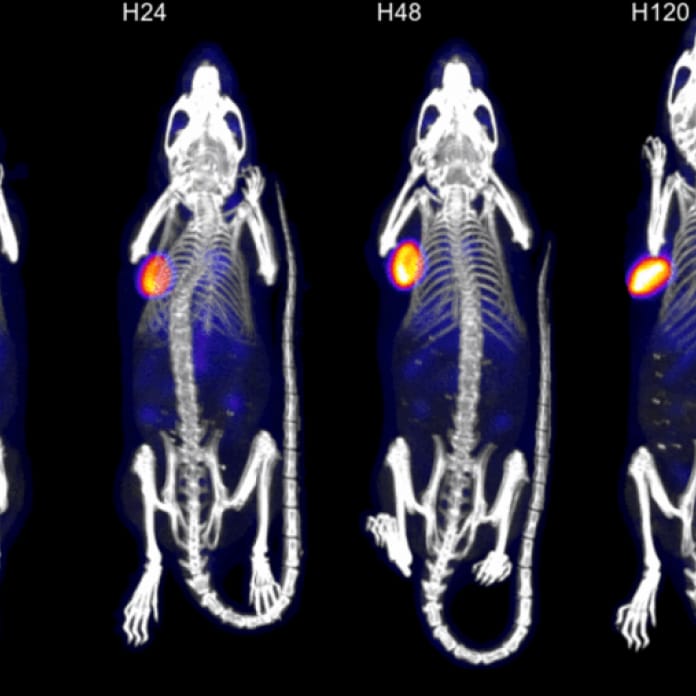
- Preclinical proof-of-concept studies – biodistribution, efficacy, toxicology
- Good manufacturing practice (GMP) radiochemistry development and production support for imaging agents