PSMA-PET Imaging Solutions
Perceptive is an industry-leading CRO providing full-service PSMA-PET solutions from radiochemistry development to preclinical and Phase 0-IV clinical trials. We support researchers that are developing PSMA-targeted prostate cancer therapeutics or multi-specifics, and groups using PSMA expression as a biomarker for patient selection, and treatment response in prostate cancer clinical trials. Perceptive advances PSMA-PET standard of care by combining advanced image analytics and artificial intelligence with standard PET SUV readouts to ensure you are receiving the most meaningful data from your images. In recent years, Perceptive has overseen more clinical PSMA-PET scans (>3,600) than most other imaging CROs and is one of the only organizations to support an FDA submission combining AI and quantitative PET.
Our full-service PSMA-PET solutions include:
-
- Complete global core lab support for Phase 0-IV clinical trials
- Radiology expertise for study design, image analysis, and sub-specialty reads (PCWG3)
- Advanced analytical tools for whole body image quantification to estimate disease burden, lesion localization, and response to therapy
- Radiochemistry development and expert guidance on PSMA ligand access and utilization, including external manufacturing support
- Preclinical solutions for discovery research
- Extensive experience with Regulatory filing support (e.g., IND-enabling support)
Perceptive’s clinical team has expertise in medical imaging and a vast range of tools to support your cancer drug discovery and development needs. With over 200 scientists and 4,800+ qualified imaging centers and clinical sites across the globe, Perceptive has the advanced scientific, medical, and regulatory expertise and project management scale to deliver studies from Phase 0-IV. Our technology platforms support key decision-making in:
- First-in-human, single-site clinical trial support
- Late-stage, global multi-center clinical trial support with response criteria reads
- Advanced image analytics including dosimetry
- Safety profile and therapeutic efficacy studies
- Study design and consultation
- Criteria-based centralized independent reviews and internal analysis
Our imaging core lab offers criteria-driven reads for standard-of-care projects and our state-of-the-art in-house software enables 3D region-of-interest generation to support quantitative analysis methods, such as PERCIST. We also partner with subspecialist independent readers that focus on specific areas of expertise. Some of the validated criteria examples include:
- RECIST 1.0/1.1
- PCWG 2.0/3.0
- RANO
- LUGANO
- IMWG
- CHESON
- iRECIST
- irRECIST
- LYRIC
- irRC
Quantitative Oncology Image Analytics
Perceptive’s medical image analysis team supports standard analysis of FDG PET imaging, including PERCIST and other SUV-based metrics, metabolic tumor volume, and total lesion glycolysis. The image analysis team also specializes in developing and applying a broad range of tools to support advanced quantitative oncology tracer characterization and analytics across a broad range of modalities and studies. Some examples include:
- Powerful, multi-modal tumor and organ Segmentation tools
- A range of flexible Biomathematical Modeling tools
- Dosimetry calculations across radionuclide, administration route, and species
- Integrated Radiomics pipelines using standard and custom feature vectors
- Preclinical and clinical support for Tracer Characterization
- Sophisticated batch Image Triage and preprocessing methods
For more information, check out some of our most popular case studies. A full list is also available in our case studies section.
Discovery and Preclinnical Capabilities
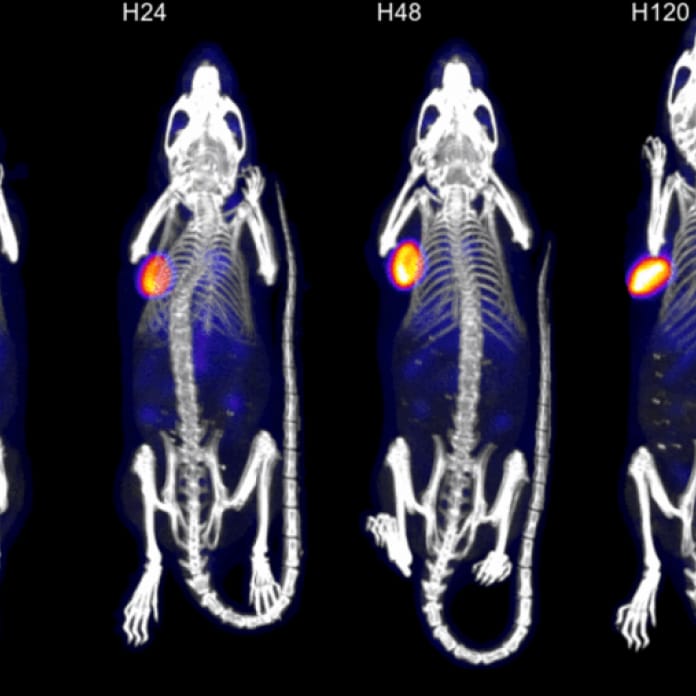
Perceptive’s discovery and preclinical services team provides study design, study execution, and data analysis services for a broad range of solid and non-solid tumor studies. Our preclinical oncology team uses state-of-the-art imaging techniques to characterize the behavior of novel therapies, including antibodies, radioligand therapies, peptides, cell therapies (e.g., CAR-T), exosomes, and other tumor-targeting modalities in diverse tumor model types (e.g., subcutaneous, orthotopic, metastatic). Non-clinical work supported by Perceptive can be used to move into first-in-human studies to late-stage clinical multicenter trials. Our core capabilities include the measurement of multiple readouts, including:
- Biodistribution of test articles
- Target density/engagement
- Pharmacokinetics (PK)
- Pharmacodynamics (PD)
- Tumor growth properties
- Therapeutic efficacy
- Dosimetry
Perceptive’s radiochemistry team has experience with most commercially available radioisotopes used for PET/SPECT imaging and several used in targeted radiotherapy. Our capabilities span from first-in-human clinical studies to GMP implementation of novel radiotracers, including: